

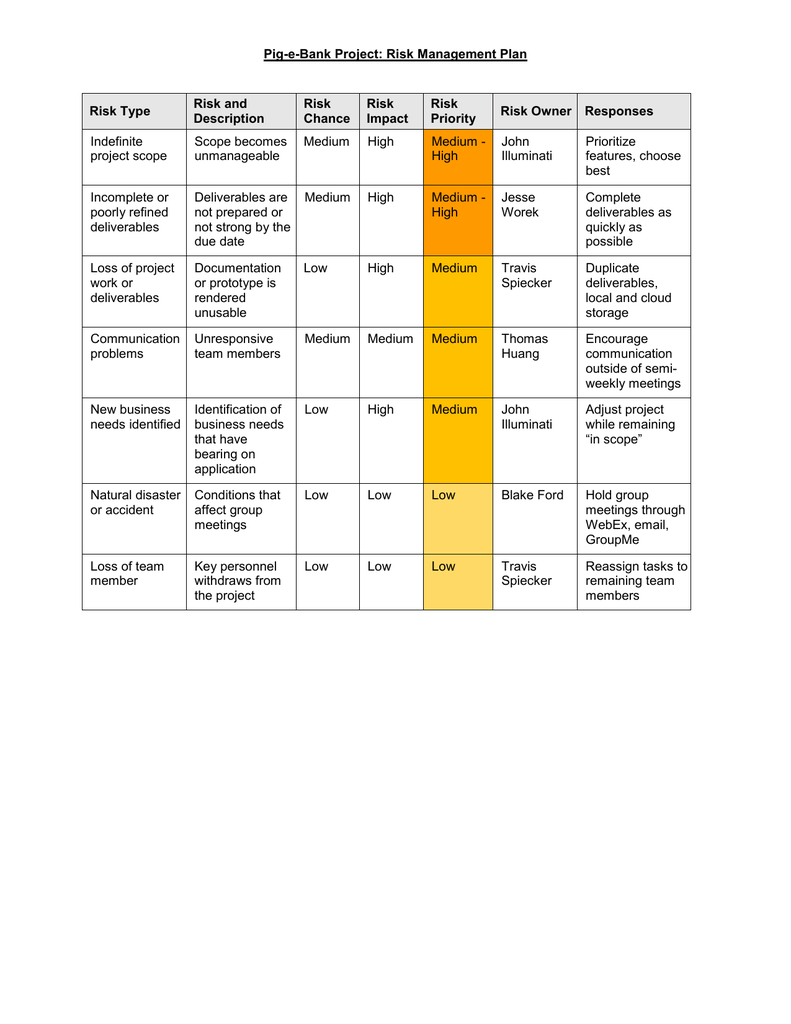
1) Safety specification 2) Pharmacovigilance activities 3) Risk minimization activities. The RMP consists of the following three elements for individual drugs.
#Risk management plan code
The YouTube videos are available directly from the QR code below. (Videos are available only in Japanese.) ≪How to view the e-learning videos≫(only on the Japanese site)
The set has been available since March 2020 on the PMDA’s YouTube channel and on its website.
#Risk management plan how to
PMDA, in collaboration with the Japanese Society of Drug Informatics and with the intention of enhancing the promotion of utilization of RMP in clinical settings, has developed an e-learning video set titled “Simple Enough to Start today! How to use RMP” as an easy-to-understand guide. Simple Enough to Start Today! How to use RMP RMP and RMP Materials have been posted on the PMDA website, information has been provided through the PMDA Medi-navi, and “Learn about RMP in 3 Minutes,” an RMP made-easy material, as well as “Simple Enough to Start Today! How to use RMP,” have been prepared and released. PMDA has made efforts to promote utilization of RMP and RMP Materials in clinical settings. Contents of RMP and RMP Materials were confirmed at PMDA and the RMP Marking is affixed to these materials to indicate that they are materials prepared in line with RMP. Such materials are prepared for drugs for which additional information provided by materials and other means was considered necessary in the process of marketing authorization review. In the risk minimization activities, besides the routine risk minimization activities such as providing information through electronic package inserts, materials (the RMP Materials) may be prepared intended for medical professionals and patients as additional risk minimization activities. In addition, to address these risks or missing information, collection of the missing information, examination of risk factors for important identified risks and causality assessment of important potential risks (the pharmacovigilance activities) or post marketing activities such as information provision for risk minimization (the risk minimization activities) are described. It also lists adverse events for which a relationship to the administered drug is suspected but not thoroughly verified (important potential risks) and identifies the information considered insufficient (important missing information) for predicting the post-marketing safety of the drug. RMP lists already confirmed ADRs (important identified risks).

Sharing the published information among medical professionals is meant to ensure further enhancements of post-marketing safety measures. The RMP aims that the risks of drugs are evaluated at regular intervals or in response to the progress of post-marketing surveillance and a set of pharmacovigilance activities to minimize the risks of drugs. The RMP is a document which shows the consistent risk management of drug from the development phase to the post-marketing phase. In order to ensure the safety of drugs, it is important to assess measures for appropriate management of the risks of drugs at any time from the development phase to the regulatory review and the post-marketing phase.


 0 kommentar(er)
0 kommentar(er)
